For decades, scientists viewed fibroblasts as little more than the structural scaffolding of the body. These cells, the most common in connective tissue, were thought to simply provide support. However, recent academic research is fundamentally changing this view, revealing that fibroblasts possess a hidden power to actively heal the brain after a stroke. These dynamic cells are now emerging as key players in the brain’s natural repair process, offering a beacon of hope for developing new therapies that could one day significantly improve outcomes for stroke survivors.
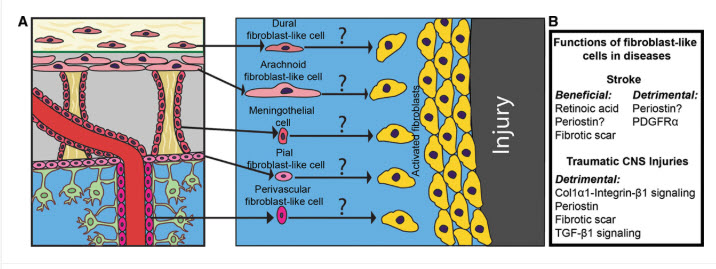
After a stroke, the brain’s delicate environment is compromised. This is especially true of the blood-brain barrier; a protective sheath that prevents harmful substances from entering the brain. In the event of a stroke, this barrier can become ‘leaky’, and fibroblasts rush to the scene to serve as the brain’s ‘plumbers’. This is where their true nature shines through. Instead of being passive support cells, fibroblasts spring into action, traveling from larger vessels to the damaged capillaries to patch up the breach in the blood brain barrier. A recent study found that these fibroblasts secrete a protein called TIMP2, a critical tool for repairing the damage and restoring the barrier’s integrity… and that by shoring up the blood brain barrier, fibroblasts help mitigate further damage and create a more stable environment for healing.
The fibroblast’s healing role doesn’t stop at mending the barrier. These cells create protective scars that stabilise the damaged tissue. This fibrotic scar tissue was once thought to be purely inhibitory to recovery, but studies show it serves a dual purpose. Early on, the scar is essential for providing structural integrity and containing inflammation. However, fibroblasts are also key to orchestrating a delicate balancing act; after the initial wound is contained, they transition to new roles, moderating the inflammatory response to ensure it doesn’t cause more harm.
In a fascinating sequence of events, some fibroblasts recruit immune cells needed for repair, while others regulate inflammation. Some even return to their original locations in the protective membranes surrounding the brain. This orchestrated and timed response suggests a sophistication previously not associated with these cells.
While much of this research is still in its early stages and based on animal models, it opens up exciting new avenues for treatment. Understanding the distinct stages of fibroblast activity, from early wound-healing to late-stage immune modulation, could guide the timing of new interventions. For example, therapies that enhance the early, beneficial scarring might be used immediately following a stroke, while those that modulate the later immune response could be used in the chronic phase.
Researchers are also exploring whether drugs already used for other fibrotic conditions, such as lung and liver fibrosis, could be adapted for brain injuries. In the long term, scientists are even exploring the possibility of directly injecting beneficial proteins like TIMP2 into the injured brain, bypassing the need for the body’s own fibroblasts to deliver them. This new understanding of fibroblasts is more than just a biological curiosity; it offers a compelling vision for the future of stroke treatment…



One Comment
i’m recovering from a stroke in june 2024 and doing research which i would like to get funding for . Neuroplasticity is amazing