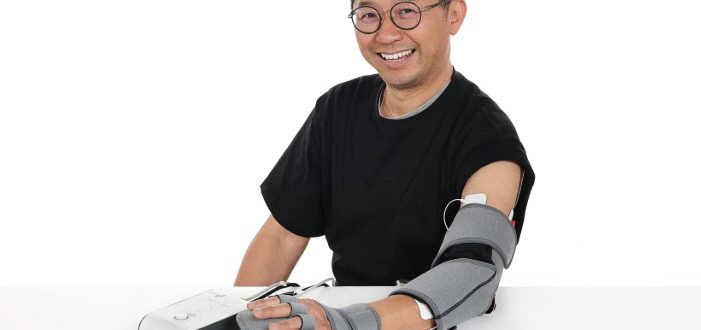
For many stroke survivors, regaining the use of their arm and hand is a significant challenge on the road to recovery. Intensive, repetitive and personalised therapy is key, but can be difficult to access consistently. Thecon Technology (HK) Limited, founded by researchers from The Hong Kong Polytechnic University (PolyU), has developed an innovative solution to this problem: the Mobilexo Arm. This wearable hybrid system combines robotics and functional electrical stimulation (FES) to accelerate and assist upper limb rehab.
The Mobilexo Arm is a portable, three-in-one rehab instrument for the paretic upper limb, designed to be used both in clinical settings and by patients at home, Unlike older FES devices with static patterns, the Mobilexo Arm uses electromyography (EMG) to detect residual electrical signals in the user’s muscles. This allows patients to control the device with their own intent, strengthening the brain’s feedback loop and encouraging neuroplasticity. It incorporates hybrid soft, inflatable components that provide mechanical assistance to the elbow, wrist and fingers. This offers a more natural and comfortable alternative to the heavy, rigid robotic arms often found in clinics.
The device provides targeted Neuromuscular Electrical Stimulation (NMES) to activate and strengthen muscles, improving coordination and sensory awareness in the affected limb. An accompanying mobile app allows patients and therapists to monitor progress remotely, track rehabilitation data in real-time, and access a variety of gamified exercises, making training more engaging and effective.
As a cutting-edge medical device, the Mobilexo Arm is not currently available for direct purchase by individuals in the UK. Thecon Technology’s current business model focuses on partnerships with hospitals and clinics. The commercialisation path for such devices typically involves extensive regulatory approvals, so its availability in the UK market would require passing these stringent tests first.
While a specific price for the UK has not been announced, the Mobilexo Arm Pro version is listed at a substantial cost in other regions. However, reports have indicated that the company is also working on a cheaper, home-based version for direct purchase by patients. For now, interested patients in the UK would need to inquire with their local stroke rehabilitation centres or explore participation in clinical trials if they become available.
The Mobilexo Arm’s main advantage lies in its hybrid design and EMG-driven control. By combining the strengths of FES and robotics into one wearable device, it offers a more sophisticated, responsive, and comfortable therapy experience.
The active participation required from the patient, guided by their own muscle signals, is far more effective at promoting neuroplasticity and functional recovery than passive, pre-set stimulation. The portability and engaging app-based exercises also make it easier for survivors to perform the frequent, high-intensity training crucial for regaining upper limb function, extending their rehabilitation beyond the clinical setting.