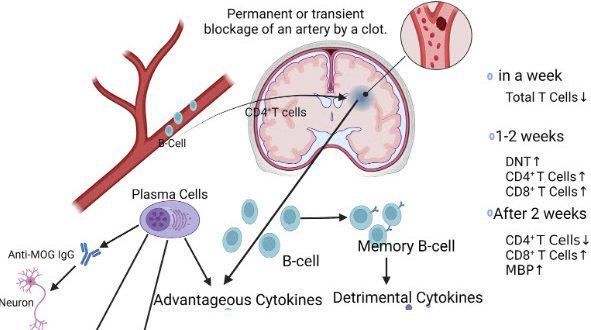
A critical research breakthrough from the Universities of Manchester and Edinburgh, recently published in the journal Brain, Behavior and Immunity, has finally provided a definitive biological explanation for the heightened vulnerability many survivors experience following a neurological event. The study reveals that a stroke triggers a significant and measurable depletion of B cells, the specialised immune cells responsible for producing antibodies to neutralise pathogens. This systemic immune exhaustion means that the body is left without its primary defensive toolkit, explaining why post-stroke patients are statistically more likely to suffer from secondary infections such as pneumonia or urinary tract infections which frequently derail the intensive rehabilitation process.
The implications of this B-cell deficiency extend far beyond simple infection risk as they provide a clear link to the profound and persistent fatigue that often stalls physical recovery efforts. When the immune system is compromised, the body diverts immense metabolic energy towards maintaining basic defences, leaving less fuel for the high-intensity task-specific practice required for neuroplasticity. For a serious survivor, this data confirms that post-stroke exhaustion is a systemic physiological reality rather than a psychological hurdle. Understanding that the body is operating with a weakened shield allows for a more strategic approach to training, emphasising the need for meticulous infection control and optimised nutrition to protect the remaining immune landscape.
This research marks a pivot in how we must view long-term stroke management, moving away from a localised focus on the brain towards a holistic understanding of systemic health. By pinpointing the loss of B cells, scientists have opened the door for future targeted therapies that could potentially replenish these vital cells or boost antibody production during the high-risk window of recovery. Until such treatments are clinical realities, survivors must act as their own first line of defence, recognising that a crash in energy may be a sign of the immune system struggling to cope. Managing these biological variables is just as essential as physical gym work, as a healthy immune system provides the stable foundation necessary to sustain the relentless pursuit of functional independence.