
Approximately 70% of stroke survivors experience a weakened arm immediately after the stroke and for 40%, this persists beyond 6 months1. Arm weakness can have a very large effect on the individual’s routine daily activities such as eating, dressing, washing, cleaning, and shopping and can also reduce potential employability.
Stroke survivors have identified arm weakness as a high priority for clinical research which aims to produce better functional outcomes in the upper limb2. Current physical therapies are limited in their success and are also very time demanding. Therefore, effective augments to use alongside rehabilitation are sought.
In a recent trial3 it was shown that stimulating the vagus nerve (VN) whilst carrying out rehabilitation exercises led to better arm recovery compared to the control group (no stimulation – rehabilitation only). However, this trial used a surgically implanted VN stimulator which required surgery under general anaesthesia and, the rehabilitation therapy was mostly delivered in hospital.

Now a new national groundbreaking multi-centre trial called TRICEPS, led by Professor Arshad Majid and researchers from the Sheffield Teaching Hospitals NHS Foundation Trust, is investigating whether arm recovery after stroke can be improved by using a non-invasive VN stimulator.
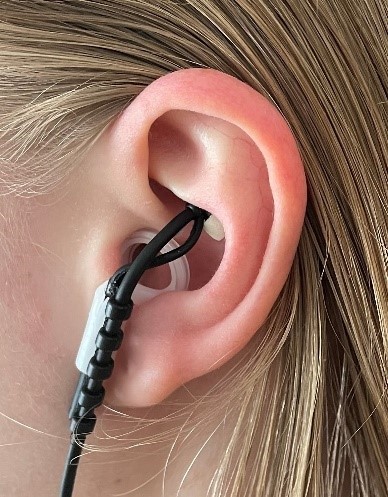
The trial uses a device which is worn as an earpiece (image 1 and 2), whilst self-delivered rehabilitation exercises are carried out at home. Surgery is not needed as a branch of the VN, located within the ear, is stimulated through the skin (Transcutaneous Vagus Nerve Stimulation, TVNS).
Using brain scanning and blood tests the trial also aims to explore how TVNS helps the brain to repair its function after stroke.
How can I get involved?

 Sheffield Teaching Hospitals and NHS Trusts nationally are looking for stroke survivors (aged 18+) with persistent arm weakness following an ischaemic stroke, which occurred between 6 months and 10 years ago.
Sheffield Teaching Hospitals and NHS Trusts nationally are looking for stroke survivors (aged 18+) with persistent arm weakness following an ischaemic stroke, which occurred between 6 months and 10 years ago.
The trial involves wearing a TVNS earpiece and wristband (Image 1 and 2) whilst doing rehabilitation therapy at home. Some participants will also be asked to wear the device while performing their usual daily activities.
If YOU would like to join in, you’ll be asked to attend a face-to-face appointment at their nearest research centre at the start of the trial, followed by two further appointments at 3 and 6 months.
What support will there be?
- Participants will be given specific instructions regarding the device and the rehabilitation exercises.
- The 12-week rehabilitation therapy plan will be tailored specifically to each participant.
- A member of the clinical research team will organise phone or video calls with participants throughout the 12-week treatment period.
- If required, these may be completed face-to-face at the research facility.
- Some research centres are able to offer home visits.
- Participants will be reimbursed for travel costs incurred as part of the trial.
 I am interested, where can I take part?
I am interested, where can I take part?
The trial is open at 19 NHS centres across England and Wales.
A full list is available on the trial website here www.triceps-trial.com
How do I express interest?
Please contact the central research team in Sheffield who will carry out a quick preliminary assessment of eligibility by phone and refer you onto your nearest research site.
Central TRICEPS Research Team contact:
Email: triceps@sheffield.ac.uk
Phone: 07935 514510


- https://www.nice.org.uk/guidance/ng236/chapter/Recommendations#intensity-of-stroke-rehabilitation
- French et al., “Repetitive task training for improving functional ability after stroke,” 2016, doi: 10.1002/14651858.CD006073.pub3.www.cochranelibrary.com.
- Dawson, J ∙ Liu, CY ∙ Francisco, GE ∙ et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial 2021; 397:1545-1553





5 Comments
is this available in India
is this available in India
i had a cerbal infarct june 2019, i have recovered well, however i have quite a prominent tremor in my left hand, luckily i am right handed……i also have a pacemaker
I would like to be considered for the trial, I had a right side hemorrhage in 2011. But I have also had a defibrillator fitted in February this year, so I know I can not use any FES near my chest. Is this system OK to use if so I like to try as I’m unhappy with my arm and hand.
Kind regards.
Joe.
My husband was on this trial for 12 weeks in April and the results in that 12 weeks were phenomenal! Just one hour a day of repetitive task training for 5 days a week for 12 weeks. He went from not being able to open his hand to pick anything up, to picking up something as small as a blueberry. In my opinion, this is the ultimate device!