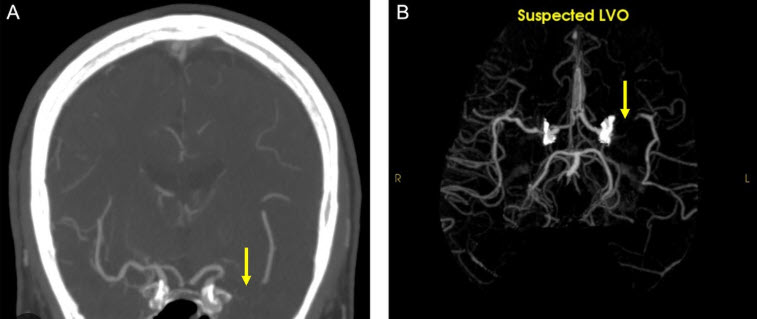
If you’ve had a stroke and like engaging with nature, Izzy and Rocksy, two friends of ARNI Stroke Rehab UK (Occupational Therapy students from the University of Southampton), would love to hear from you as they’re doing a study on how people with neurological conditions like stroke experience and engage with nature-based activities, such as gardening, outdoor walks, training in nature, conservation projects etc… please help them!
If you’ve had a stroke and like engaging with nature, Izzy and Rocksy, two friends of ARNI Stroke Rehab UK (Occupational Therapy students from the University of Southampton), would love to hear from you as they’re doing a study on how people with neurological conditions like stroke experience and engage with nature-based activities, such as gardening, outdoor walks, training in nature, conservation projects etc… please help them!
Nature-based rehabilitation (NBR) delivered as part of neurorehabilitation is a brilliant and developing approach.
To help the Southampton University students do research and create a protocol, do read on and consider doing a quick, helpful computer-based interview (no need to go anywhere) with them if you can spare the time!

The Southampton students would like to understand better, things like:
- What does nature mean to you?
- What helps or prevents you to engage with it?
- How does it support or challenge your wellbeing and daily life?
As you know, each year in the UK, around 100,000 people experience a stroke contributing to a community of approximately 1.3 million stroke survivors, more than half of whom live with a disability.
 Post-stroke fatigue is one of the most common and challenging symptoms to manage. Dong et al, (2025), reported that 45.8 % of individuals in their study experienced post-stroke fatigue, highlighting the need for interventions that address depression, improve quality of life and restore activities of daily living.
Post-stroke fatigue is one of the most common and challenging symptoms to manage. Dong et al, (2025), reported that 45.8 % of individuals in their study experienced post-stroke fatigue, highlighting the need for interventions that address depression, improve quality of life and restore activities of daily living.
Living with a neurological condition, such as stroke, involves navigating a complex mix of physical, cognitive and emotional challenges that affect everyday life, wellbeing and connection with others. Many people experience fatigue, anxiety, reduced confidence or difficulty taking part in activities that once felt meaningful.
 As interest grows in holistic, person-centred approaches to rehabilitation, nature engagement is emerging as a promising way to support wellbeing for individuals living with long-term conditions.
As interest grows in holistic, person-centred approaches to rehabilitation, nature engagement is emerging as a promising way to support wellbeing for individuals living with long-term conditions.
Nature engagement is endless and broad, and it can range from a forest walk, gardening, horticultural engagement, horse riding and many more possibilities!
Research suggests that spending time in natural environments can offer meaningful benefits such as improvements in mood, confidence, social connection, and overall quality of life for people with neurological conditions. Many people describe nature as a space where they feel more motivated, more independent and more able to challenge themselves in their rehabilitation journey.
To date, despite all these findings, there remains limited research exploring the personal experiences of nature engagement for people with neurological conditions.
Izzy and Rocksy want to contribute to stroke survivors – they state that ‘understanding these perspectives is essential for developing accessible, effective and meaningful nature-based programmes that actually reflect what stroke survivors value and need’.

This study aims to gather the experiences of people who have experienced stroke and who take part in any form of nature-based activity.
The Southampton students would like to understand better, things like:
- What nature means to you?
- What helps or prevents you to engage with it?
- How does it support or challenge your wellbeing and daily life?
 Your insights can help shape future stroke rehabilitation approaches and ensure that nature-based programmes are designed with lived experience at their core.
Your insights can help shape future stroke rehabilitation approaches and ensure that nature-based programmes are designed with lived experience at their core.
Your insights will help us understand what makes it easier or harder to take part in these kinds of activities and how they can be made more accessible for everyone.
 What will happen to me if I take part?
What will happen to me if I take part?
- If you agree to take part, we will ask you to fill in a consent form and a short form to provide background information about you.
- You will be invited to a one-to-one interview lasting around 45–60 minutes. This will take place online via Microsoft Teams at a time that suits you.
- During the interview, we will ask you about your experiences with nature-based activities, the factors that make participation easier or harder, and what you feel the benefits are.
- The interview will be video recorded on Microsoft teams (with your consent) to help us accurately transcribe what is said. The recording will be securely deleted once the transcription is complete. We will remove any identifying information about you from the transcript.
- This transcript will be securely stored on a University of Southampton SharePoint site, accessible to the research team. You do not need to answer any questions that make you feel uncomfortable. You can take breaks or stop the interview at any time..
 Are there any benefits in my taking part?
Are there any benefits in my taking part?
Yes! Your views will contribute to raising awareness and improving understanding of how nature-based interventions are experienced and delivered.
The Southampton students hope that your participation will help inform future practice and promote greater inclusivity for people living with long term neurological conditions. Please contact them on either im2g23@soton.ac.uk or ra11g23@soton.ac.uk
The findings from this study will also be used to teach undergraduate students about the value and application of nature based programmes.
The aim is to gain insight into barriers and enablers that impact engagement in nature-based programmes, and to understand how these activities can be improved and sustained to better support the wellbeing of people with long-term neurological conditions.
The project is not funded in any way.
* Researchers: Izzy Mason, Undergraduate Researcher (BSc Occupational Therapy) University of Southampton and Rocksy Antonygnaneswaran, Undergraduate Researcher (BSc Occupational Therapy), University of Southampton.
* Supervisor: Long-time friend of ARNI Stroke Rehab UK Charity: Dr Leisle Ezekiel (lezekiel@soton.ac.uk)
Dong, Y., Tang, L., Salwismawati Badrin, Salziyan Badrin and Wu, J. (2025). Factors associated with post-stroke fatigue among stroke survivors: a cross-sectional study. PeerJ, 13, pp.e19052–e19052.







 Post-stroke fatigue is one of the most common and challenging symptoms to manage. Dong et al, (2025), reported that 45.8 % of individuals in their study experienced post-stroke fatigue, highlighting the need for interventions that address depression, improve quality of life and restore activities of daily living.
Post-stroke fatigue is one of the most common and challenging symptoms to manage. Dong et al, (2025), reported that 45.8 % of individuals in their study experienced post-stroke fatigue, highlighting the need for interventions that address depression, improve quality of life and restore activities of daily living. As interest grows in holistic, person-centred approaches to rehabilitation, nature engagement is emerging as a promising way to support wellbeing for individuals living with long-term conditions.
As interest grows in holistic, person-centred approaches to rehabilitation, nature engagement is emerging as a promising way to support wellbeing for individuals living with long-term conditions.
 Your insights can help shape future stroke rehabilitation approaches and ensure that nature-based programmes are designed with lived experience at their core.
Your insights can help shape future stroke rehabilitation approaches and ensure that nature-based programmes are designed with lived experience at their core. What will happen to me if I take part?
What will happen to me if I take part? Are there any benefits in my taking part?
Are there any benefits in my taking part?