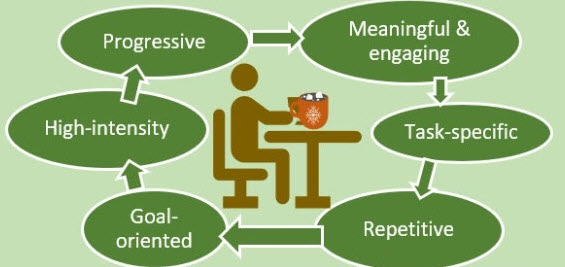
 Upper limb impairment is a common and persistent consequence of stroke, significantly affecting an individual’s independence and quality of life. The evidence shows clearly that the cornerstone of effective motor recovery is task-specific practice, a principle underpinned by evidence from neuroscience and motor learning theory. This approach posits that the brain re-organises itself in response to intensive and repetitive functional training, fostering neuroplasticity.
Upper limb impairment is a common and persistent consequence of stroke, significantly affecting an individual’s independence and quality of life. The evidence shows clearly that the cornerstone of effective motor recovery is task-specific practice, a principle underpinned by evidence from neuroscience and motor learning theory. This approach posits that the brain re-organises itself in response to intensive and repetitive functional training, fostering neuroplasticity.
In the private and home rehabilitation settings, stroke survivors can engage in task-specific practice very easily by themselves or with the help of an ARNI Stroke Rehab UK trainer or physio. The rationale is that by repeatedly and intensively practicing meaningful, real-world activities, from lifting a cup to getting dressed, the brain reorganises neural pathways, improving the control over motor functions relevant to those specific tasks.
 This approach moves beyond general exercises to focus directly on the functional skills that enhance independence, motivating patients through visible, goal-oriented progress. While private clinics can offer the benefit of therapist supervision, specialised equipment and intensive regimens, stroke survivors reading this will be all too aware that consistent practice at home is crucial for achieving high dosages of repetition rates necessary for effective motor learning. ARNI Stroke Rehab UK instructors work with patients to set up personalised routines, often leveraging accessible technology or adapted household items, making rehabilitation a continuous, integrated part of daily life.
This approach moves beyond general exercises to focus directly on the functional skills that enhance independence, motivating patients through visible, goal-oriented progress. While private clinics can offer the benefit of therapist supervision, specialised equipment and intensive regimens, stroke survivors reading this will be all too aware that consistent practice at home is crucial for achieving high dosages of repetition rates necessary for effective motor learning. ARNI Stroke Rehab UK instructors work with patients to set up personalised routines, often leveraging accessible technology or adapted household items, making rehabilitation a continuous, integrated part of daily life.
The evidence base for task-specific practice is strong, and it ranks highly among recommended interventions. Moreover, national and international guidelines, such as those from the UK’s Royal College of Physicians and NICE recommend repetitive task training to improve upper limb weakness.
Correspondingly, multiple systematic reviews and meta-analyses over the years consistently report that repetitive task training yields positive, sustained improvements in mobility and upper limb function, whether implemented soon or long after a stroke. While some studies note that the effect size can be small, its efficacy often eclipses that of traditional, less focused approaches. The robust body of evidence supports task-specific practice as a cornerstone of modern stroke rehabilitation, validating its widespread use by therapists in both clinical and home environments.
 For task-specific practice to be effective, it should be relevant to the survivor’s goals, performed frequently, and incorporate feedback to reinforce learning. However, traditional-type therapy has been evidenced to struggle to provide the sheer volume of high-quality repetitions needed to drive meaningful neural recovery. Correspondingly, a range of technologies have emerged fill this need to assist and optimise task-specific practice.
For task-specific practice to be effective, it should be relevant to the survivor’s goals, performed frequently, and incorporate feedback to reinforce learning. However, traditional-type therapy has been evidenced to struggle to provide the sheer volume of high-quality repetitions needed to drive meaningful neural recovery. Correspondingly, a range of technologies have emerged fill this need to assist and optimise task-specific practice.
But stroke survivors shouldn’t view upper limb stroke rehab as a magic bullet. Tech can be an invaluable assistor, providing tools and methods to enhance and intensify these exercises, making high-repetition, focused practice more accessible and engaging. But only by using tech as an assistor to hard work, will the tech do its job. In other words task-specific training can be helped by technology but you need to get into a self rehab training programme first and understand that tech won’t work to increase function and action control after stroke without countenancing hard work.
 Clinics can now days employ some very sophisticated robotic and electromechanical systems to maximise task-specific training, with many devices in the range of the average stroke survivor’s pocket. Some of these devices are robotic exoskeletons that provide adjustable arm weight support, allowing individuals with severe weakness to perform a greater range of movement. The principle of gravity compensation enables survivors to initiate and control movements themselves, rather than being passively moved, which is crucial for neuroplasticity.
Clinics can now days employ some very sophisticated robotic and electromechanical systems to maximise task-specific training, with many devices in the range of the average stroke survivor’s pocket. Some of these devices are robotic exoskeletons that provide adjustable arm weight support, allowing individuals with severe weakness to perform a greater range of movement. The principle of gravity compensation enables survivors to initiate and control movements themselves, rather than being passively moved, which is crucial for neuroplasticity.
For instance, ANYexo (left) is an advanced, versatile robotic exoskeleton designed for upper limb rehab in stroke survivors, enabling high-repetition training for a broad range of abilities, from survivors who are severely to those mildly affected. It features a unique kinematic structure for near-human arm movement, series elastic actuators for precise force control and intuitive programming for a wide array of daily living activities. Due to its cost it’s aimed usually at clinical settings.
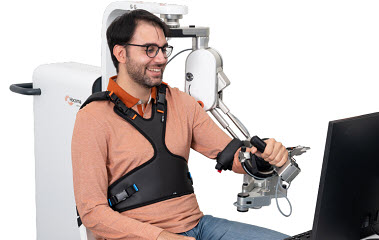 For instance the Hocoma ArmeoSpring Pro (right) is a robotic exoskeleton system which provides adjustable arm weight support for the entire movement chain, from the shoulder to the hand, through a patented technology. This counterbalances gravity, allowing individuals with severe weakness to perform a greater range of movement.
For instance the Hocoma ArmeoSpring Pro (right) is a robotic exoskeleton system which provides adjustable arm weight support for the entire movement chain, from the shoulder to the hand, through a patented technology. This counterbalances gravity, allowing individuals with severe weakness to perform a greater range of movement.
The device is used in conjunction with virtual reality exercises to make therapy engaging and functional. The principle is to enable intensive, high-repetition practice of arm and hand movements in a controlled and supportive environment. The system encourages patients to initiate and control movements themselves, with the robotic arm providing just enough assistance to complete the task. This active engagement is vital for neuroplasticity.
But for stroke survivors wanting to optimise rehab at home, several other wearable and consumer-focused devices are currently available in the UK. For example, robotic gloves can offer assisted and passive training via powered mechanisms to aid grasping and releasing movements. The principle behind these home-use devices is to enable intensive, self-directed practice, leveraging biofeedback and gamification to promote plasticity. Some of these employ gamified exercises to allow users to perform high-repetition arm and hand movements, addressing the limitation of traditional therapy by increasing the dose and intensity of training outside of rehab sessions.
 For instance, the Neofect Smart Glove (left), is a soft, wearable hand-and-wrist rehabilitation device that uses gamified exercises to improve motor function which incorporates sensors that track movements of the wrist and fingers, providing a platform for therapy with accompanying software offering a variety of games targeting different movements and abilities…
For instance, the Neofect Smart Glove (left), is a soft, wearable hand-and-wrist rehabilitation device that uses gamified exercises to improve motor function which incorporates sensors that track movements of the wrist and fingers, providing a platform for therapy with accompanying software offering a variety of games targeting different movements and abilities…
Another tool is a powered orthosis like the Myomo MyoPro (below) that uses electromyography (EMG) sensors to detect residual muscle signals, activating a motor to assist with arm and hand movements based on the user’s intent.
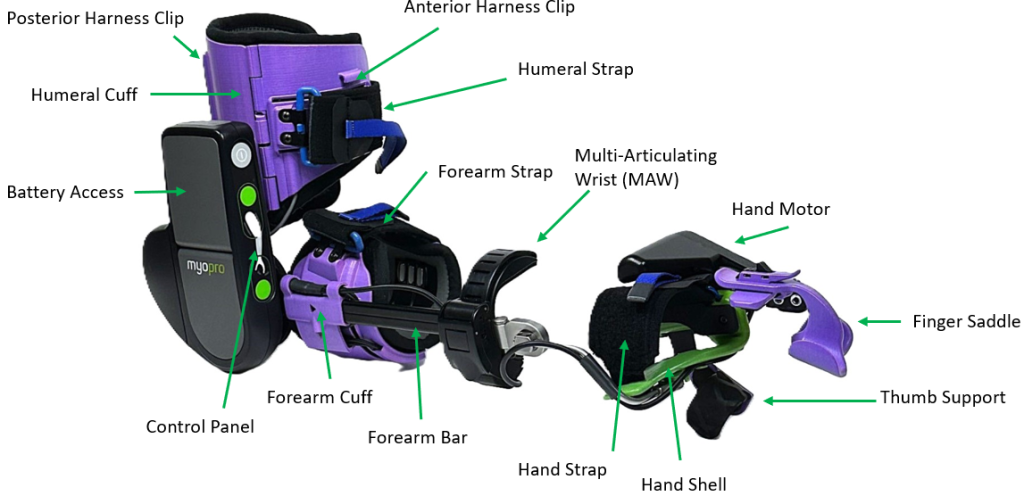 The principle here is to leverage a survivor’s own bio-signals to drive movement, creating a powerful biofeedback loop that promotes active participation and self-initiation of movement.
The principle here is to leverage a survivor’s own bio-signals to drive movement, creating a powerful biofeedback loop that promotes active participation and self-initiation of movement.
Like the Hocoma device, this is not a product for direct consumer purchase; rather, it’s a specialist clinical device accessed through expert rehabilitation providers. The costs for these advanced systems are substantial, reflecting their complexity and clinical application.
Functional electrical stimulation (FES) is another core technology used by therapists, sometimes integrated into other systems: FES for the upper limb delivers mild electrical stimulation to targeted muscles via a cuff, assisting with grasp and release.
 The principle is to provide an external impetus for muscle contraction, which, when paired with the stroke survivor’s intent during a functional task, strengthens the neural pathways controlling movement. This helps re-educate the neuromuscular system and can enable the ability to perform task-specific practice. Clinical access to such FES systems is available through NHS and private rehabilitation services, with pricing depending on the clinical package.
The principle is to provide an external impetus for muscle contraction, which, when paired with the stroke survivor’s intent during a functional task, strengthens the neural pathways controlling movement. This helps re-educate the neuromuscular system and can enable the ability to perform task-specific practice. Clinical access to such FES systems is available through NHS and private rehabilitation services, with pricing depending on the clinical package.
An alternative, manual approach used by therapists for survivors with severe impairment is known as finger-equipped electrode electrical stimulation (FEE-ES) (left) which allows them to apply FES with precise manual control, synchronising the stimulus with even subtle, intended movement. This technique does requires a trained therapist to do it.
 An example of a wearable for the home market is the Bioness H200 Wireless; (right) a sleek, wireless FES device that delivers mild electrical stimulation to specific arm and hand muscles via electrodes integrated into a soft cuff. The stimulation is controlled via an intuitive handheld unit or app, allowing for functional, task-specific training. The core principle is that FES provides an external impetus for muscle contraction, which, when paired with the patient’s intent to move, strengthens the neural pathways controlling arm and hand function.
An example of a wearable for the home market is the Bioness H200 Wireless; (right) a sleek, wireless FES device that delivers mild electrical stimulation to specific arm and hand muscles via electrodes integrated into a soft cuff. The stimulation is controlled via an intuitive handheld unit or app, allowing for functional, task-specific training. The core principle is that FES provides an external impetus for muscle contraction, which, when paired with the patient’s intent to move, strengthens the neural pathways controlling arm and hand function.
Repetitive, intentional practice using the H200 helps re-educate muscles, reduce spasticity, and increase range of motion. In the UK, the Bioness H200 is available through private rehabilitation clinics, such as ARNI colleagues PhysioFunction and Hobbs Rehabilitation. It’s also supplied through specialist distributors like Summit Medical and Scientific. Access is typically via a clinical assessment, followed by a trial and supervised training. Pricing is substantial and depends on the specific package and clinical support required.
 Others incorporating FES and EMG which are designed for survivors to purchase, like the Nura-FES, (left) are also being designed (Nura-FES is patent-pending and under investigation by long-term friend of ARNI, Professor Cherry Kilbride at Brunel University).
Others incorporating FES and EMG which are designed for survivors to purchase, like the Nura-FES, (left) are also being designed (Nura-FES is patent-pending and under investigation by long-term friend of ARNI, Professor Cherry Kilbride at Brunel University).
Unlike 25 years ago, when I had my own stroke, there is now a diverse range of technologies (from sophisticated clinical robots to consumer-friendly wearables) which can now support task-specific practice for upper limb stroke rehab. We are seeing more and more therapists (in clinical settings) able to utilise high-end robotic exoskeletons and FES systems to deliver intensive, assisted training in a controlled clinical environment, leveraging principles like gravity compensation, EMG-driven movement, biofeedback and VR to promote high-repetition re training.
Combining therapist-guided sessions with appropriate home-based wearables offers a comprehensive approach to maximising rehabilitation potential and improving functional independence. While access to high-end robotic systems remains primarily within specialist centres, more affordable wearables and digital platforms are democratising access to intensive rehabilitation, empowering survivors to take an active role in their recovery.
This burgeoning integration of wearable technology and other innovative systems within upper limb stroke rehabilitation is pretty much reshaping clinical practice and home-based recovery for UK stroke survivors. And research indicates that these technological interventions enable a higher dosage and intensity of task-specific practice, a critical driver of plasticity that has been to date limited by finite personal or clinical resources. This technological shift is not only augmenting the capacity of therapists but also offering a pathway toward more personalised, effective, and accessible rehabilitation, with the ultimate goal of improving functional independence and long-term outcomes for a larger population of stroke survivors.
For a more detailed look at new products for upper limb stroke rehab, please access ARNI Stroke Rehab UK News Channel and browse through the posts.







 Upper limb impairment is a common and persistent consequence of stroke, significantly affecting an individual’s independence and quality of life. The evidence shows clearly that the cornerstone of effective motor recovery is task-specific practice, a principle underpinned by evidence from neuroscience and motor learning theory. This approach posits that the brain re-organises itself in response to intensive and repetitive functional training, fostering neuroplasticity.
Upper limb impairment is a common and persistent consequence of stroke, significantly affecting an individual’s independence and quality of life. The evidence shows clearly that the cornerstone of effective motor recovery is task-specific practice, a principle underpinned by evidence from neuroscience and motor learning theory. This approach posits that the brain re-organises itself in response to intensive and repetitive functional training, fostering neuroplasticity. This approach moves beyond general exercises to focus directly on the functional skills that enhance independence, motivating patients through visible, goal-oriented progress. While private clinics can offer the benefit of therapist supervision, specialised equipment and intensive regimens, stroke survivors reading this will be all too aware that consistent practice at home is crucial for achieving high dosages of repetition rates necessary for effective motor learning. ARNI Stroke Rehab UK instructors work with patients to set up personalised routines, often leveraging accessible technology or adapted household items, making rehabilitation a continuous, integrated part of daily life.
This approach moves beyond general exercises to focus directly on the functional skills that enhance independence, motivating patients through visible, goal-oriented progress. While private clinics can offer the benefit of therapist supervision, specialised equipment and intensive regimens, stroke survivors reading this will be all too aware that consistent practice at home is crucial for achieving high dosages of repetition rates necessary for effective motor learning. ARNI Stroke Rehab UK instructors work with patients to set up personalised routines, often leveraging accessible technology or adapted household items, making rehabilitation a continuous, integrated part of daily life. For task-specific practice to be effective, it should be relevant to the survivor’s goals, performed frequently, and incorporate feedback to reinforce learning. However, traditional-type therapy has been evidenced to struggle to provide the sheer volume of high-quality repetitions needed to drive meaningful neural recovery. Correspondingly, a range of technologies have emerged fill this need to assist and optimise task-specific practice.
For task-specific practice to be effective, it should be relevant to the survivor’s goals, performed frequently, and incorporate feedback to reinforce learning. However, traditional-type therapy has been evidenced to struggle to provide the sheer volume of high-quality repetitions needed to drive meaningful neural recovery. Correspondingly, a range of technologies have emerged fill this need to assist and optimise task-specific practice. Clinics can now days employ some very sophisticated robotic and electromechanical systems to maximise task-specific training, with many devices in the range of the average stroke survivor’s pocket. Some of these devices are robotic exoskeletons that provide adjustable arm weight support, allowing individuals with severe weakness to perform a greater range of movement. The principle of gravity compensation enables survivors to initiate and control movements themselves, rather than being passively moved, which is crucial for neuroplasticity.
Clinics can now days employ some very sophisticated robotic and electromechanical systems to maximise task-specific training, with many devices in the range of the average stroke survivor’s pocket. Some of these devices are robotic exoskeletons that provide adjustable arm weight support, allowing individuals with severe weakness to perform a greater range of movement. The principle of gravity compensation enables survivors to initiate and control movements themselves, rather than being passively moved, which is crucial for neuroplasticity. For instance the Hocoma ArmeoSpring Pro (right) is a robotic exoskeleton system which provides adjustable arm weight support for the entire movement chain, from the shoulder to the hand, through a patented technology. This counterbalances gravity, allowing individuals with severe weakness to perform a greater range of movement.
For instance the Hocoma ArmeoSpring Pro (right) is a robotic exoskeleton system which provides adjustable arm weight support for the entire movement chain, from the shoulder to the hand, through a patented technology. This counterbalances gravity, allowing individuals with severe weakness to perform a greater range of movement. For instance, the Neofect Smart Glove (left), is a soft, wearable hand-and-wrist rehabilitation device that uses gamified exercises to improve motor function which incorporates sensors that track movements of the wrist and fingers, providing a platform for therapy with accompanying software offering a variety of games targeting different movements and abilities…
For instance, the Neofect Smart Glove (left), is a soft, wearable hand-and-wrist rehabilitation device that uses gamified exercises to improve motor function which incorporates sensors that track movements of the wrist and fingers, providing a platform for therapy with accompanying software offering a variety of games targeting different movements and abilities… The principle here is to leverage a survivor’s own bio-signals to drive movement, creating a powerful biofeedback loop that promotes active participation and self-initiation of movement.
The principle here is to leverage a survivor’s own bio-signals to drive movement, creating a powerful biofeedback loop that promotes active participation and self-initiation of movement. The principle is to provide an external impetus for muscle contraction, which, when paired with the stroke survivor’s intent during a functional task, strengthens the neural pathways controlling movement. This helps re-educate the neuromuscular system and can enable the ability to perform task-specific practice. Clinical access to such FES systems is available through NHS and private rehabilitation services, with pricing depending on the clinical package.
The principle is to provide an external impetus for muscle contraction, which, when paired with the stroke survivor’s intent during a functional task, strengthens the neural pathways controlling movement. This helps re-educate the neuromuscular system and can enable the ability to perform task-specific practice. Clinical access to such FES systems is available through NHS and private rehabilitation services, with pricing depending on the clinical package. An example of a wearable for the home market is the Bioness H200 Wireless; (right) a sleek, wireless FES device that delivers mild electrical stimulation to specific arm and hand muscles via electrodes integrated into a soft cuff. The stimulation is controlled via an intuitive handheld unit or app, allowing for functional, task-specific training. The core principle is that FES provides an external impetus for muscle contraction, which, when paired with the patient’s intent to move, strengthens the neural pathways controlling arm and hand function.
An example of a wearable for the home market is the Bioness H200 Wireless; (right) a sleek, wireless FES device that delivers mild electrical stimulation to specific arm and hand muscles via electrodes integrated into a soft cuff. The stimulation is controlled via an intuitive handheld unit or app, allowing for functional, task-specific training. The core principle is that FES provides an external impetus for muscle contraction, which, when paired with the patient’s intent to move, strengthens the neural pathways controlling arm and hand function.  Others incorporating FES and EMG which are designed for survivors to purchase, like the
Others incorporating FES and EMG which are designed for survivors to purchase, like the 